Development
Home | Services & Solutions | Drug Product | Parenteral | Development
The parenteral pre-formulation, formulation and analytical teams are located in Shanghai Waigaoqiao site and Wuxi city site, supporting 60+ compounds in 2023 from preclinical studies through clinical trials to commercial launch, spanning all synthetic molecules, including small molecules, oligonucleotides, peptides and complex chemical conjugates.
In addition, we also have high potency R&D labs for drug product with OEL limit of 10 ng/m3.
We support all types of dosage forms and filling formats.
Drug Types
- Small molecule
- Oligonucleotide
- Peptide
- Synthetic conjugate
Dose Forms
- Solution / emulsion
- Suspension
- Lyophilized powder
- Advanced formulation, e.g., liposome / LNP
Filling Formats
- Vial
- Prefilled syringe
- Cartridge
Various Products
- Intramuscular / subcutaneous
- Intravenous
- Intrathecal
- Inhalation
- Ophthalmic
Pre-formulation Study
Our developability and formulation research supports your parenteral drugs from “Hit to Lead”, through “Lead Optimization” until “Candidate Selection” with a comprehensive physical-chemical characterization with minimal amount of API required.
We have a team of 50+ experienced scientists who complete over 60,000 characterization tests every year. Our facilities are equipped with 40+ types of instruments, totaling over 170. This enables us to handle a large number of projects simultaneously.
Our tests include:
Physical form characterization
- Crystalline / amorphous
- Tm, Tg, PI
- Water / solvent content
Power property characterization
- Particle size
- Morphology
- Hygroscopicity
Solubility study
- In pH buffer
- In-situ salt
- Pharmaceutical vehicles (co-solvent, surfactant, CDs…)
Stability study
- Bulk stability
- Solution stability (acidic, basic, oxidation, light…)
- Compatibility
Our high throughput formulation screening, enabled by automatic compound weighing, vehicle screening and high throughput analysis, can complete sample preparation, stability study, analysis, optimization until lead formulation selection within 2 weeks, while typical industry timeline is about 4 weeks.
Formulation Development
Dosage forms
- Normal solution
- Lyophilized power
- LNP / PNP
- Micelle
- Others
Route of Administration
- Intravenous
- Subcutaneous
- Ophthalmic
- Intrathecal
- Others
Highlight of our formulation development capabilities
- Flexible batch size
- Organic solvent compounding & hydrophobic filtration (e.g. DMSO)
- Extreme low fill volume
- High viscosity mixing and filling
- Elevated or cold temperature mixing
- Dissolved oxygen and headspace control
- Sterilization methods
- Sterile filtration / moist heat sterilization
- Irradiation (outsource)
We are experienced in all stages including multiple phase III and commercial projects with 100% success rate of PPQ campaigns.

Integrated CMC Solution
Our integrated CMC solution includes both API and formulation development and manufacturing for injectable drugs. Through streamlined processes and parallel activities among different teams within WuXi STA, we can accelerate your programs with the most efficient CMC solution.
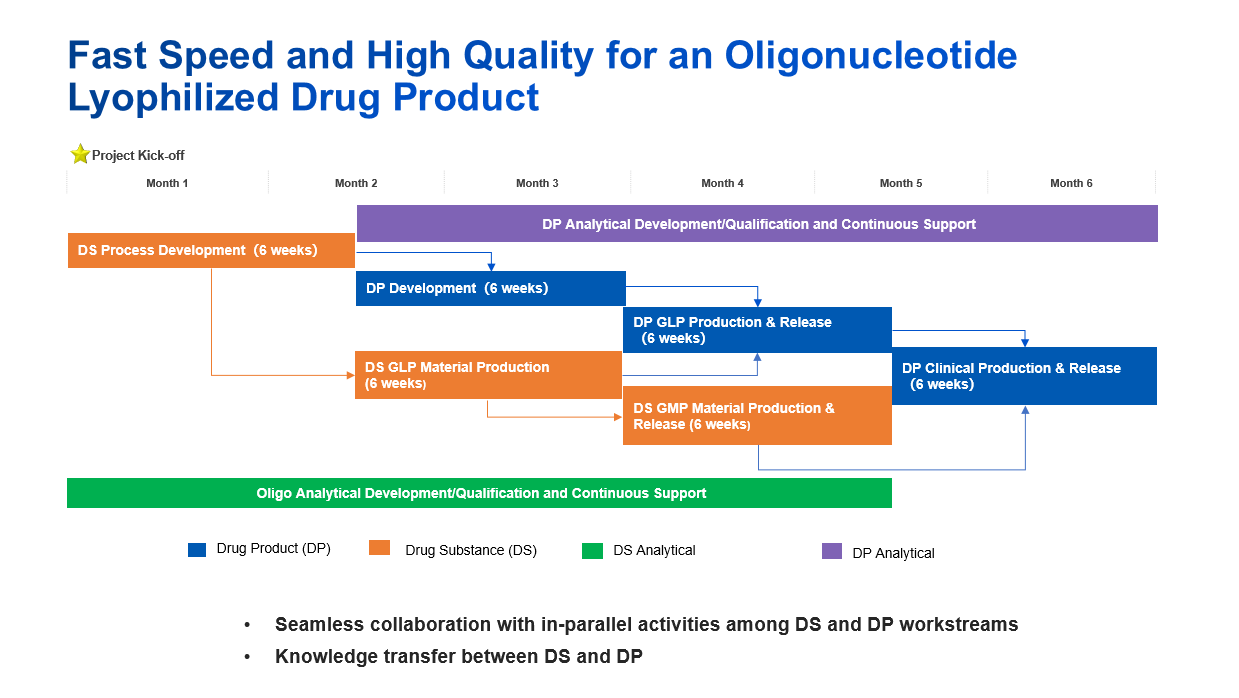
Case Study 1: Fast Speed and High Quality for an Oligonucleotide Lyophilized Drug Product
In this case study, we developed an oligonucleotide lyophilized drug product within 6 months from API process development to clinical trial supply by seamless collaboration with in-parallel activities among drug substance, drug product and analytical workstreams.
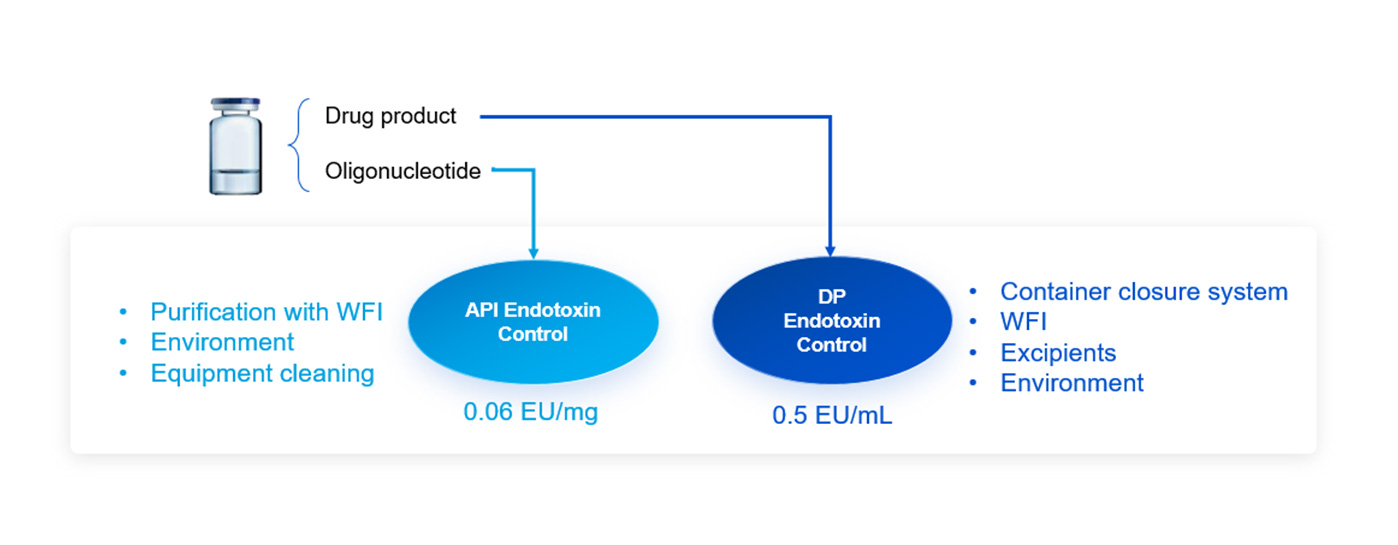
Case Study 2: Endotoxin Control of an Intrathecal Injectable Oligonucleotide Drug
It is a case study that we developed an oligonucleotide intrathecal (IT) injectable drug for IND application to the US FDA in sterile solution. It required very low endotoxin level. Our API team successfully controlled the endotoxin level to 0.06 EU/mg and our drug product team developed the clinical trial supply for phase I with endotoxin level at 0.5 EU/mL.
Lipid Nanoparticle Platform
The LNP R&D lab is equipped with multi-channel micro-mixing system, different scales of Tangential flow filtration (TFF) system, sterile filtration system and analytical equipment. Other mixing technologies are also available, such as MIVM, microfluidics, Precision Nano System and LNP extrusion systems. LNP feasibility studies, formulation development and process development are carried out in R&D labs. The professional teams with extensive experience can accelerate the projects from R&D to GMP manufacture. Read more.