Home | Services & Solutions | Drug Product | Oral | Solid State Development
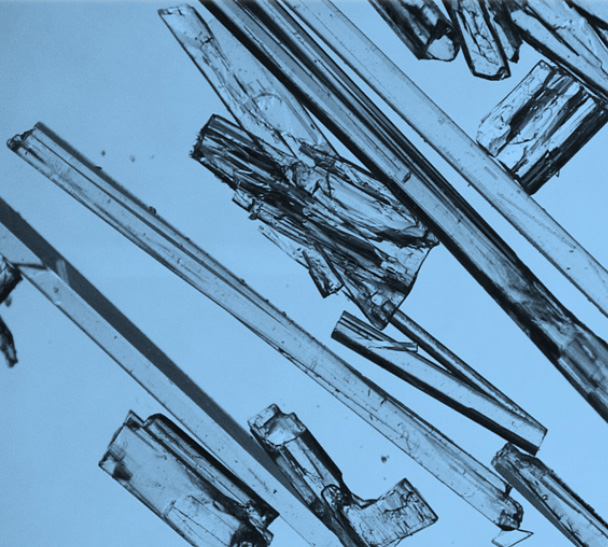
Solid-state properties for oral solid drugs can significantly impact their pharmaceutical performance. A thorough characterization of solid-state behavior of the API and drug product is critical to the success of the drug development at all stages.
Our experts in solid state chemistry, powder characterization, and material science are dedicated to accelerating your drug substance and drug product development from preclinical to commercial manufacturing. With more than 90 dedicated scientists, we conduct 400+ solid form screening projects at all development stages every year. We also support high potency compound with OEL of 10ng/m3.
90+
Scientists
70,000+
Characterization Tests Annually
1,000+ m2
Lab Space
400+
Projects Annually
30+
Advanced
Instruments
Support Entire New Drug Development Life Cycle
for Both Drug Substance and Drug Product
Discovery
Preclinical
Phase I
Phase II
Phase III
Commercial
Solid State Chemistry
Salt/cocrystal screening and polymorph screening for tox and clinical development
Comprehensive polymorph study for NDA filing
Salt and polymorph study for technical life cycle management
Solid state characterization
Solid form quantitative method development
Crystal structure analysis
Simulation and modeling
Trouble shooting
Characterization
Flowability | Particle size | Morphology | Surface area | Density
Material Science
SEM-EDS | Micro-CT | Raman | Wettability | Texture analysis
Discovery
Preclinical
Solid State Chemistry
Salt/cocrystal screening and polymorph screening for tox and clinical development
Solid State Characterization
Crystal Structure Analysis
Phase I
Phase II
Solid State Chemistry
Comprehensive polymorph study for NDA filing
Solid form quantitative method development
Simulation and Modeling
Phase III
Commercial
Salt and Polymorph study for Technical Life Cycle Management
Solid form quantitative method development
Trouble shooting
Characterization
Flowability | Particle Size | Morphology | Surface Area | Density
Material Science
SEM-EDS | Micro-CT | Raman | Wettability | Texture Analysis
Capabilities
- Solid form screening and analysis: salt and crystal form screening, co-crystal screening, polymorph relationship investigation, solid form developability assessment and single crystal analysis
- Solid state characterization: flowability, particle size, morphology, surface area and density
- Solid form quantitative method development
- High potency compound solid form screening and characterization
Various cutting-edge equipment to support general and specific testing
- X Ray Powder Diffraction (XRPD) with a Cryo-RH chamber
- Capillary X Ray Powder Diffraction (XRPD)
- DSC/mDSC and nano DSC (Differential Scanning Calorimetry)
- Thermogravimetric Analysis (TGA)
- Dynamic Vapor Sorption (DVS)
- Fourier-Transform Infrared Spectroscopy (FTIR)
- Confocal Raman
- Scanning Electron Microscopy (SEM) and Energy Dispersive X-ray Spectroscopy (EDS)

Pave an Expressway to Phase I – Strategies that Accelerate Your Early Phase Programs
The success of any new drug development starts in its early phase. The probability of success of a molecule increases dramatically after it passes first-in-human (FIH) and proof-of-concept (POC) studies. Building a comprehensive understanding of the molecule is crucial to successful early phase development. API salt form and formulation selections are also key factors to successful phase I studies. Effective CMC strategies can significantly enhance the speed of early phase development and ultimately the chance for success in late-stage development. This webinar will present our experience in using the integrated API and drug product platform and formulation technologies to accelerate early-phase development to phase I and beyond. Access on-demand webinars & videos In this webinar, we will discuss the importance of phase-appropriate process development and various